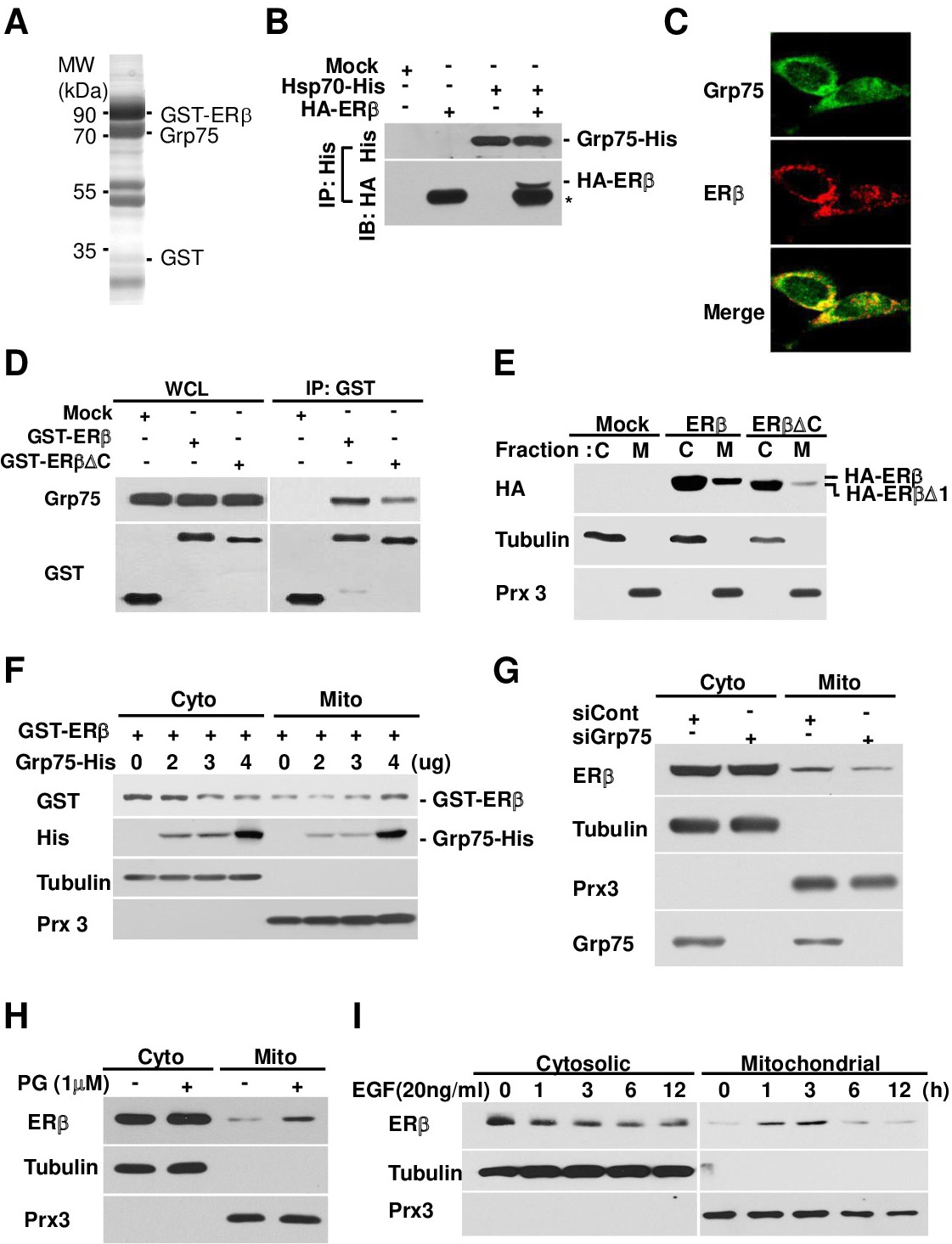
Fig. 2. ERβ interacts with Grp75 protein and translocates into mitochondria. A. ERβ interacting protein was affinity-purified using GSH-Sepharose beads and separated on a 10% denaturing gel. The Coomassie-stained gel shows the proteins identified by MALDI-TOF mass spectrometry and the presence of ERβ and Grp75 in the purified complex. B. MCF7 cells were transfected with pHA-ERβ and pcDNA-Grp75-his, cell extracts were immunoprecipitated with anti-His antibody as described in the Materials and Methods section, and the precipitated proteins were subjected to immunoblotting. C. MCF7 cells were stained with the indicated antibodies and observed by confocal microscopy. D. Protein-protein interactions of ERβ or ERβ△C with Grp75 in the indicated plasmid-transfected MCF7 cells. WCL, whole cell lysate. E. MCF7 cells were transfected with an ERβ- or ER△C-expressing plasmid, followed by separation into cytosolic (C) and mitochondrial (M) fractions and immunoblotting with the indicated antibodies. F. MCF7 cells were co-transfected with pEBG-GST-ERβ and pcDNA-Grp75 plasmid at the indicated doses, and separated into cytosolic (C) and mitochondrial (M) fractions. G. MCF7 cells were transfected with siRNA against the Grp75 gene or control, separated into cytosolic (C) and mitochondrial (M) fractions, and subjected to immunoblotting. H and I. MCF7 cells were treated with progesterone (PG; H) for 1 hour or epidermal growth factor (EGF; I) for the indicated times, separated into cytosolic (C) and mitochondrial (M) fractions, and subjected to immunoblotting with the indicated